Join the Fight
Against COVID-19
From the start of the COVID-19 pandemic, researchers have been working to develop a vaccine against this disease. Help us to test a future vaccine candidate against COVID-19.

If your child is 12-15 years old, please click the button below to complete the study pre-screen questionnaire.
If you are 16-17 years old, please click the button below to complete the study pre-screen questionnaire.
What Happens If You/Your Child Are/Is Accepted?
After being accepted to take part in this study, you/your child will be randomly assigned to one of two groups: the first group includes participants receiving the VLA2001 COVID-19 vaccine candidate and those in the second group will initially receive a placebo. Our study will admit at least 660 participants who will be split equally (1:1). 330 adolescents will receive VLA2001 and the remaining 330 participants will initially get the placebo only, followed by the VLA2001 vaccine candidate two months after enrolment.
You/your child will not know which treatment is given and neither will the study doctor.

330
VLA2001

330
Placebo
After an initial screening visit, the participant will need to visit the site another seven or eight times (possibly more on request), depending on which treatment group you are assigned. Study-related activities will include physical examinations, COVID-19 testing, and blood and urine samples. In total, participation is required for approximately 15 months.

Now Looking For Healthy Participants
There are several COVID-19 vaccines, both approved and in development. Each one uses a different technology to prevent people from getting sick after being infected by the virus. Valneva’s Phase 3 clinical trial is currently testing a new vaccine candidate against COVID-19 in adults. The study population is now extended to include adolescent participants and this information is intended for adolescents and parents or guardian of potentially eligible participants.
You/your child can participate in this trial to confirm the safety of Valneva’s vaccine candidate and test its ability to induce antibodies as a protection against the virus.
What to Expect:
- Everyone who participates in this study will receive either the VLA2001 vaccine candidate or a placebo (a substance that has no therapeutic effect and is used as a control in testing new drugs).
- For participants in the VLA2001 group, the vaccine candidate will be delivered in two doses, 28 days apart. A third dose (booster vaccination) will be administered seven months after enrolling into the study, for a total of three injections.
- Participants in the placebo group will receive two doses of placebo, 28 days apart. Two months after enrolling into the study, they will receive two doses of the VLA2001 vaccine candidate, 28 days apart, for a total of four injections.
- The study will take 15 months to complete.
- Study participants must complete a minimum of eight or nine visits; additional visits may be required.
- Selected study participants will be compensated for their time and participation.
To be eligible to participate, you must:
- Be between 12 and 17 years old.
- Have stable health (meaning that any present condition is not likely to worsen to the point the participant is no longer able to continue the study).
- Be able to attend all study visits and maintain an e-diary for seven days after each injection.
- Agree to use highly effective contraception during participation (if applicable).
This study will investigate the level of antibodies against COVID-19 induced by the VLA2001 vaccine candidate and whether it is safe for use. We will recruit at least 660 adolescents to help us evaluate the vaccine candidate compared to a placebo.
With this study we aim at answering the following two main questions:
- Is the immune response induced by VLA2001 comparable in adults and in adolescents?
- Is the VLA2001 COVID-19 vaccine candidate safe for adolescents?

University Hospitals Bristol and Weston NHS Foundation Trust
Cheadle Hospital
St George's University Hospitals NHS Foundation Trust
Epsom and St Helier University Hospitals
COVID-19 is a disease caused by the SARS-CoV-2 coronavirus. Common COVID-19 symptoms include fever, cough, dyspnoea (shortness of breath), muscle and body ache, fatigue, loss or change of smell and taste, as well as diarrhea. Serious complications or death are possible. More than 4 million people worldwide have died from COVID-19 or associated illnesses since the onset of the pandemic, out of approximately 190 million confirmed cases.
In an effort to control the spread of COVID-19, researchers around the world have been working to develop vaccines. Today, there are a few approved COVID-19 vaccines and more in development. Based on the different technologies used in their development, the COVID-19 vaccines work differently in the body.
To date, approximately 3,150 adults have received at least one dose of VLA2001 vaccine candidate in two ongoing clinical trials. An independent group of experts regularly reviews the safety information from these studies and, to date, has not identified any safety concern. Overall, the trials showed that VLA2001 vaccine candidate was safe and well-tolerated at all dose levels tested.
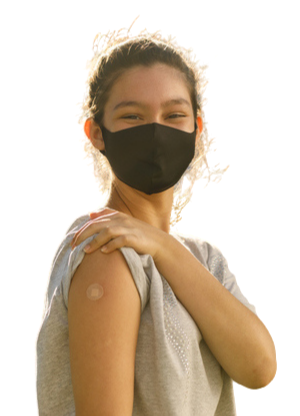
Why Should I/My Child Participate in COVID-19 Research?
COVID-19 is a global concern. Nearly 2.4% of the world population has had a confirmed case of the virus and more are being infected every day. Together, we can work to limit the spread and fight against COVID-19, protecting our families, friends, neighbours and ourselves from catching the virus.
Before enrolment in the COVID-19 vaccine study, here are some things to consider:
Pros:
- Participants will receive a COVID-19 vaccination (either with a vaccine candidate, or with a placebo at the initial stage of the study and then the vaccine approximately 2 months after enrolment.
- The progress of all participants will be supported every step of the way.
- Your participation could help others.
Cons:
- Participants in the placebo-group will not receive the vaccine candidate immediately.
- The candidate vaccine may not be as effective as the licensed COVID-19 vaccines.
- You/your child could experience side effects, such as headache, fatigue, muscle pain, or injection site tenderness.
Clinical studies are how drugs, treatments, and vaccines are studied to ensure that they are safe and effective before they become available to the public. Participants volunteer to receive an investigational product (or, in this case, a vaccine candidate), meaning a product currently being researched. Then, they are checked by medical staff to see how they react to that substance. Researchers look at those results and determine whether the product works and if it is safe.
Every single vaccine, prescription drug, or therapeutic treatment on the market today went through a series of clinical studies before it became part of modern healthcare. It truly takes a team, and it all starts with study participants or volunteers.
Large teams work together. Volunteers participate, giving their time and their bodies. Researchers work with test subjects to make sure the prevention or treatment is safe and effective. Investigators analyse results, run lab tests, and follow up with participants for months, sometimes years, after they stop receiving doses of the investigative product. Regulators review those findings before ultimately granting the drug or vaccine approval.
The vaccine candidate being tested in this study, VLA2001, is called an investigational vaccine because it is currently being investigated or studied before possible approval for public use by health authorities.
One group will receive VLA2001 vaccine candidate and the other group will receive placebo (a substance that has no therapeutic effect and is used as a control in testing new drugs), followed by the VLA2001 vaccine candidate at a later time.
No. It is impossible to get COVID-19 from a COVID-19 vaccine.
While the vaccine candidate does hold some of the same germs that cause COVID-19, they are weakened and made inactive so that they cannot make you sick. Instead, your body produces antibodies against the inactivated virus. This way, if you are exposed to the virus that causes COVID-19, those antibodies are able to attack the virus, and you may not get sick.
The vaccine in this trial is an investigational vaccine, which means that the exact side effects or the commonalities of those issues have yet to be determined.
We expect that some participants will experience typical vaccination site reactions such as pain, swelling, tenderness, or redness at the injection site. In addition, there is a risk of systemic adverse reactions like headache, myalgia (muscle pain), tiredness, nausea, arthralgia (joint pain), malaise (a general feeling of being unwell), fever (high temperature), feverishness, or chills. Most side effects after vaccination are mild to moderate and will resolve within a few days of vaccination.
As with all investigational studies, the study vaccine and study procedures in this investigation may involve unknown risks. All medications can have both temporary and permanent side effects and can cause unforeseen adverse reactions.
You/your child can quit at any time. There is no penalty and no reason has to be given.
Your (child’s) information will be provided to the participating NHS site, and they will get in touch directly to discuss the study and aspects of participation. Again, participation can be stopped at any time. There is no penalty, and no reason has to be given.
It is possible that, during the course of this study, participants will become eligible, through the national vaccination roll-out, to receive an approved, nationally deployed COVID-19 vaccine. If this happens, options can be discussed with the study doctors to make an informed choice.
